Vanda Pharmaceuticals Inc. VNDA faced a setback as the FDA issued a complete response letter rejecting its new drug application (NDA) for tradipitant to treat gastroparesis.
The company’s stock took a hit, declining by 6.1% on September 19 in response to the news.
Gastroparesis, a condition characterized by delayed gastric emptying, lacks effective treatment options, with the FDA not approving any new medications for over four decades.
Year to date, Vanda’s stock has climbed 10.2%, outperforming the industry’s overall rise of 0.4%.
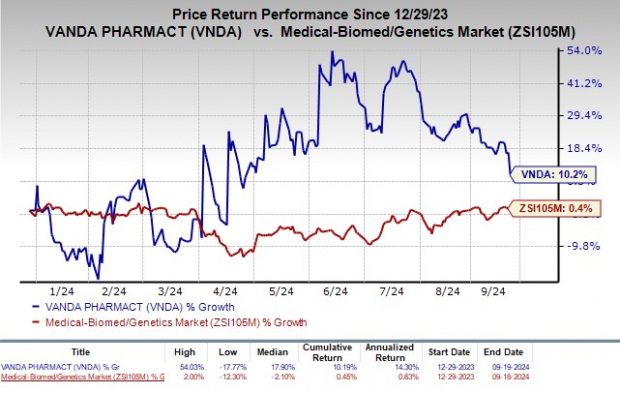
Image Source: Zacks Investment Research
FDA’s CRL for VNDA’s Tradipitant
According to the Complete Response Letter (CRL) from the FDA, Vanda was required to conduct additional studies on tradipitant, with a design that contradicted expert advice and lacked scientific basis for the disease.
Despite Vanda’s requests for an advisory committee meeting to discuss the NDA, the FDA delayed its decision by more than 185 days, breaching the Food Drug and Cosmetic Act’s timeline requirements.
Patients treated with tradipitant have submitted a Citizen Petition urging the FDA to approve the drug for gastroparesis treatment.
VNDA’s Strategy for Tradipitant
Even after the FDA rejection, Vanda remains committed to seeking marketing approval for tradipitant in gastroparesis.
Besides gastroparesis, the company is exploring tradipitant’s potential in preventing motion sickness-induced vomiting, with positive phase III trial results reported in May 2024.
Vanda aims to submit an NDA for tradipitant to the FDA for motion sickness treatment later in 2024.
Zacks Rank & Notable Stocks
Vanda currently holds a Zacks Rank #4 (Sell).
Other top-performing stocks in the biotech sector include Illumina, Inc. ILMN, Krystal Biotech, Inc. KRYS, and Fulcrum Therapeutics, Inc. FULC, all carrying a Zacks Rank #1 (Strong Buy).
Positive estimates and performance trends are seen in Illumina, with consistent earnings beats and positive analyst outlooks.
Investors monitoring Krystal Biotech and Fulcrum Therapeutics should also note the favorable adjustments in earnings estimates for the future.
Despite the recent setback, Vanda’s continued efforts in drug development reflect its resilience in pursuing innovative solutions despite regulatory challenges.